

Periodic trends in electron configuration The elements are so arranged to show periodic trends.įirst up - electron configuration. Rows are called periods, and columns are called groups. Image credits: Īll in all, the periodic table is a regular arrangement of elements by atomic number. He was proven right when these elements were later discovered. In some cases the properties didn't quite match up here, Mendeleev left gaps in the table, proposing that they would be filled by then-undiscovered elements. He therefore also arranged the elements in rows and columns, so that elements with similar properties were above and below each other in the table. Mendeleev ordered the known elements by atomic mass, but noticed that they showed certain properties which repeated every eight or so elements. The version that we use today was created in 1869 by the Russian chemist Dmitri Mendeleev, and built on the work of scientists such as John Newlands. We'll also look at density and electrical conductivity. By the end of this article, you should be able to describe and explain the trends in electron configuration, atomic radius, electronegativity, first ionisation energy, and melting and boiling points.After that, we'll explore periodic trends as you move down a group in the periodic table.We'll then take a look at periodic trends as you move along a period in the periodic table.To start with, we'll define periodicity.This article is about periodic trends in inorganic chemistry.Want to know more about these trends? Then you've come to the right place! It means that you'll find certain trends in the properties of elements, that repeat in regular intervals as you move along the table. This is because the periodic table shows periodicity.
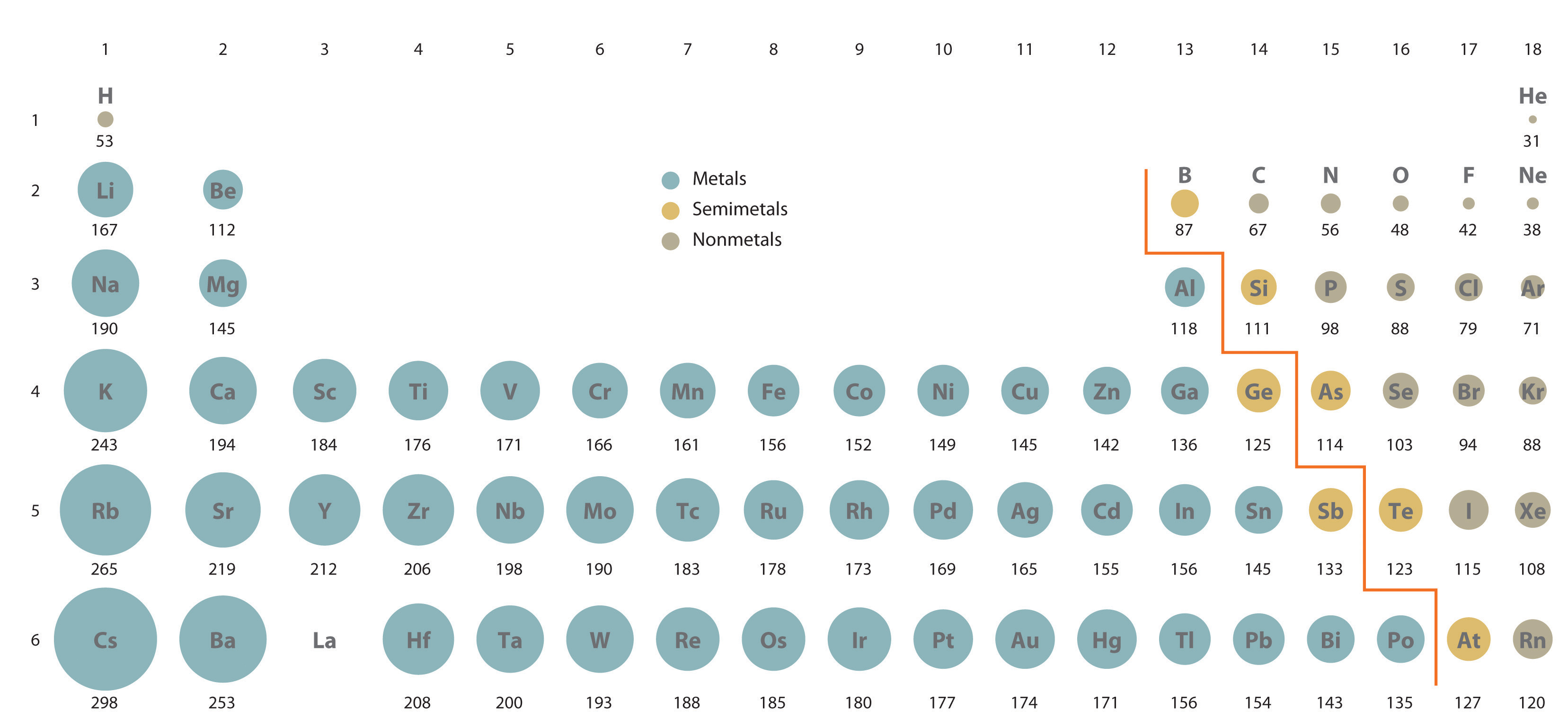
Luckily for you, this is actually the case. What if, once you knew the position of an element in the periodic table, you were able to predict its properties? Application of Le Chatelier's Principle.Variable Oxidation State of Transition Elements.Transition Metal Ions in Aqueous Solution.


 0 kommentar(er)
0 kommentar(er)
